Forensic Drug Analysis
Forensic
drug analysis deals with the identification and quantification of illegal drugs. Forensic drug tests are 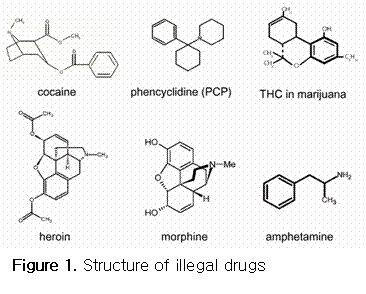 generally carried out in two steps: screening
and confirmation.1-3 Once drugs are
detected through screening, for example spot test kits (e.g., immunoassays,
Marquis test, etc), samples are then collected and sent to laboratories for
confirmation tests. Confirmation requires high sensitivity and selectivity
toward drugs, as well as their metabolites, and is frequently carried out by
GC/MS.4,5 Gas chromatography (GC) is based on
the separation of volatile samples by their unique
affinity for the column. Target drug compounds in the sample are identified by
their retention times when samples are passed through chromatographic columns.
GC coupled with mass spectrometry (MS) is a powerful technique because
structures of unknown compounds can be identified after they have been separated
by GC. Other analytical
instruments, such as HPLC, FTIR, and UV/Vis are found
in forensic laboratories as complementary techniques.6-11
generally carried out in two steps: screening
and confirmation.1-3 Once drugs are
detected through screening, for example spot test kits (e.g., immunoassays,
Marquis test, etc), samples are then collected and sent to laboratories for
confirmation tests. Confirmation requires high sensitivity and selectivity
toward drugs, as well as their metabolites, and is frequently carried out by
GC/MS.4,5 Gas chromatography (GC) is based on
the separation of volatile samples by their unique
affinity for the column. Target drug compounds in the sample are identified by
their retention times when samples are passed through chromatographic columns.
GC coupled with mass spectrometry (MS) is a powerful technique because
structures of unknown compounds can be identified after they have been separated
by GC. Other analytical
instruments, such as HPLC, FTIR, and UV/Vis are found
in forensic laboratories as complementary techniques.6-11
Despite
their proven utility, there is a continuous demand for the improvement and
optimization of current analytical techniques to detect and identify existing
and emerging illegal substances with enhanced sensitivity and selectivity.
Therefore, the following specific objectives will be pursued in this project.
1. Optimize
current analytical techniques and procedures for the detection and
identification of representative illegal drugs using GC/MS, HPLC, FTIR, and UV-Vis located in the Chemistry Department at Buffalo State
College. Although the optimization of similar instruments located at different
institutions is known in the literature,12-18 these instruments at BSC need to be prepared for
forensic research.
2. Improve
current analytical techniques by coupling individual analytical tools (e.g.,
GC/FTIR), incorporate new analytical methods for existing tools (e.g., FTIR-ART
and FTIR microscopy for drug detection at the surface), and develop new
materials for analytical purposes (e.g., stationary phases of GC and HPLC).
3. Encourage the participation of
undergraduate and graduate students in these research projects.
In this project, six drugs, marijuana (THC), cocaine, opiates (heroin and morphine), amphetamines, phencyclidine (PCP), and their metabolites, (see Figure 1 for structures) will be chosen for analysis, because they are the most frequently screened and detected in corporate and federal testing programs. The cutoff levels for each drug for regulated specimens (that is, those specimens subject to the Department of Health and Humane Service (DHHS) guidelines for Federal and Department of Transportation (DOT) workplace urine drug testing programs) are shown in the table below.
|
drugs |
cutoff level (ng/mL) |
|
|
initial test (immunoassay) |
confirmation test (GC/MS) |
|
|
amphetamines |
1000 |
500 |
|
THC (marijuana) |
50 |
15 |
|
cocaine metabolite |
300 |
150 |
|
opiates (morphine &
heroin) |
2000 |
2000 |
|
PCP |
25 |
25 |
GC (GC/MS), HPLC, FTIR, and UV/Vis will be used because they are not only the most important analytical tools in forensic drug analysis, but are also available in the Chemistry Department at Buffalo State College.
Proposed Experiments.
Phase 1. In a first set of experiments, spectroscopic and chromatographic signatures for each drug in Figure 1 will be obtained. For this goal, analytical instruments (GC/MS, HPLC, FTIR, and UV/Vis) will be optimized by the use of standard reference samples. Then, each drug compound with cutoff level will be analyzed and the results will be compared to those previously reported in the literature.12-19 The advantages and limitations of each technique will be determined. The techniques and procedures for forensic drug analysis developed in this research will be integrated into the curriculum for forensic chemistry majors.
Phase 2. In a second set of experiments, improvement in the sensitivity and selectivity of each analytical tool will be attempted.
a. GC (and GC/MS) is one of the most important analytical
tools in forensic chemistry.4,5 Although GC/MS was limited to
samples that can be capable of being volatilized in the past, solid samples can
now be analyzed via solid phase micro extraction (SPME) techniques.4,5 Improvement of GC (GC/MS)
will be conducted by developing new stationary phases and optimizing column
length.
Since separation primarily depends on interactions between drug compounds and the surface of the stationary phase, controlling surface properties (e.g., polarity, hydrophobicity, and surface functional groups) of the stationary phase is critical.20,21 Surface modification of silica, alumina, zirconia, thoria, titania, and xeolite can be achieved by forming self-assembled monolayers (SAMs) of silanes (X-(CH2)n-SiCl3, X = CH3, OH, CF3, NH2, etc). By choosing silanes with the appropriate terminal groups and chain length (X and n in X-(CH2)n-SiCl3), the surface properties of the stationary phase can be tuned and controlled. Therefore, the separation ability of GC toward individual drug compounds is expected to be improved. The surface modification of the stationary phase can be monitored by diffuse reflectance FTIR.22 Optimization of column length will also be carried out.
Secondly,
although it has already been commercialized, the analytical capabilities of GC
will be enhanced by incorporating spectroscopic features observed by FTIR.7,10 Once individual components in
a sample are separated by GC, their spectroscopic information can be monitored
by FTIR since FTIR can obtain spectroscopic data for gas phase compounds. The
sensitivity and selectivity of FTIR can be increased by the use of an MCT
(mercury cadmium telluride) detector cooled by liquid nitrogen. Therefore,
GC/FTIR will provide complementary structural information that is not available
from GC and GC/MS.
b. Although GC
is mostly used for drug detection, liquid chromatography (LC) has also gained
considerable attention.9 Improvement of LC is mainly focused on the
development of new stationary phases.
Silica
is the most widely used stationary phase support in HPLC, several problems,
however, limit their application: (1) the Si-O bond
can be broken by both acids and bases, which leads to the irreproducibility of
the chromatography and a short column lifetime; (2) exposed silanol
groups on the silica surface interact strongly with basic analytes,
cause severe peak tailing, and greatly influence selectivity.23-25 One of the ways to prevent
such problems is to cover silica surfaces by thin, dense, and hypercrosslinked organic films to ensure good HPLC
performance for basic analytes and wide pH stability.
In this project, a SAM of silanes with aromatic
groups will be prepared on the silica surface by silanization,
which can protect the silica substrate from acid and base attacks and can
shield residual silanol groups effectively.26-30
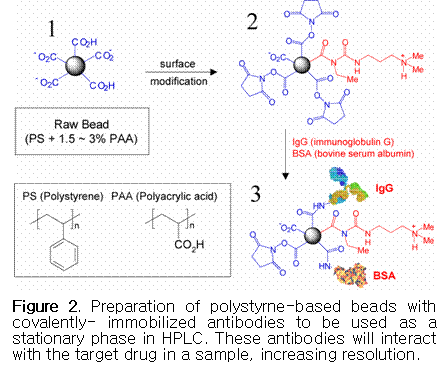 In addition, protein-modified particles are
promising stationary phases in drug separation due to their specificity and
selectivity toward target drug compounds.31,32 This type of stationary phase can be
prepared by surface modification using beads
consisting of styrene copolymerized with acrylic acid, as shown in Figure 2.
The surface can be activated by EDC (N-ethyl-N'-(3-dimethyl aminopropyl)-carbodiimide hydrochloride) and NHS (N-hydroxysuccinimide)
to immobilize proteins, drug antibodies (immunoglobulin G) and bovine serum
albumin (BSA).
In addition, protein-modified particles are
promising stationary phases in drug separation due to their specificity and
selectivity toward target drug compounds.31,32 This type of stationary phase can be
prepared by surface modification using beads
consisting of styrene copolymerized with acrylic acid, as shown in Figure 2.
The surface can be activated by EDC (N-ethyl-N'-(3-dimethyl aminopropyl)-carbodiimide hydrochloride) and NHS (N-hydroxysuccinimide)
to immobilize proteins, drug antibodies (immunoglobulin G) and bovine serum
albumin (BSA).
c. FTIR improvement will focus on surface drug analysis via application of an FTIR microscope and attenuated total reflection (ATR) mode coupled with an MCT detector.8,10 FTIR data is generally obtained by transmission mode, which requires careful isolation of the sample from the environment using standard sampling techniques. Compared to the traditional transmission mode, samples are placed on the microscope plate and FTIR data can be obtained with micrometer resolution. It has been reported that FTIR data for a single cocaine particle as small as 40 ве 30 ве 10 mm3 can be obtained.33 The other advantage in using an FTIR microscope is that two-dimensional spectroscopic images for samples can be obtained.
FTIR-ATR mode has been popularly used for structural investigations of thin film adsorbed on surfaces.8,10 By using a polarized IR source, the orientation and ordering of adsorbates can be obtained. In this work, FTIR spectra for each drug compound obtained in ATR mode will be compared to those from transmission mode. Furthermore, in situ detection of drugs in solution will be carried out using ATR crystals equipped with a flowcell.34
d. The role
played by UV/Vis in drug analysis cannot be
understated, since many illegal drugs contain aromatic groups that produce
characteristic UV/Vis spectra.11 As the absorption peak depends on the nature
of the aromatic group(s) in the drug compound, additional spectroscopic information
regarding drug derivatives and their metabolites will be obtained.
References
(1) The Analysis of Drugs of Abuse; Cole, M. D.; Caddy, B., Eds.; CRC Press: New York, 1994.
(2) Jungreis, E. Spot Test Analysis, Clinical, Environmental, Forensic, and Geochemical Applications; second ed.; John Wiley & Sons, Inc.: New York, 1997.
(3) Immunoassay, A Practical Guide; Law, B., Ed.; Taylor and Francis Ltd.: London, UK, 1996.
(4) Jack, D. B. Drug Analysis by Gas Chromatography; First Edition ed.; Academic Press, Inc.: New York, 1984.
(5) Gas Chromatography in Forensic Science; Tebbett, I., Ed.; Ellis Horwood: New York, 1992.
(6) Pharmaceutical and Biomedical Applications of Liquid Chromatography; First Edition ed.; Riley, C. M.; Lough, W. J.; Wainer, I. W., Eds.; Elsevier Science Inc.: Tarrytown, NY, 1994.
(7) White, R. Chromatography/Fourier Transform Infrared Spectroscopy and Its Applications; Marcel Dekker, Inc.: New Yourk, 1990.
(8) Practical Sample Techniques for Infrared Analysis; Coleman, P. B., Ed.; CRC Press: Boca Raton, 1993.
(9) High-Performance Liquid Chromatography in Forensic Chemistry; Lurie, I. S.; Wittwer, J., J. D., Eds.; Marcel Dekker, Inc.: New York, 1983; Vol. 24.
(10) Practical Fourier Transform Infrared Spectroscopy, Industrial and laboratory analysis; Ferraro, J. R.; Krishnan, K., Eds.; Academic Press, Inc.: New York, 1989.
(11) Ultraviolet-visible Spectrophotometry in Pharmaceutical Analysis; Görög, S., Ed.; CRC Press: New York, 1995.
(12) Smith, M. L.; Hughes, R. O.; Levine, B.; Dickerson, S.; Darwin, W. D.; Cone, E. J. J. Anal. Toxicol. 1995, 19, 18-26.
(13) Paul, B. D.; Mitchell, J. M.; Mell, L. D.; Irving, J. J. Anal. Toxicol. 1989, 13, 2-7.
(14) Cone, E. J.; Dickerson, S.; Paul, B. D.; Mitchell, J. M. J. Anal. Toxicol. 1993, 17, 156-164.
(15) Cone, E. J.; Welch, P.; Mitchell, J. M.; Paul, B. D. J. Anal. Toxicol. 1991, 15, 1-7.
(16) Cone, E. J.; Dickerson, S.; Paul, B. D.; Mitchell, J. M. J. Anal. Toxicol. 1992, 16, 72-78.
(17) Cone, E. J.; Yousefnejad, D.; Darwin, W. D.; Maguire, T. J. Anal. Toxicol. 1991, 15, 250-255.
(18) Cone, E. J.; Welch, P.; Paul, B. D.; Mitchell, J. M. J. Anal. Toxicol. 1991, 15, 161-166.
(19) Elsohly, M. A.; Little, T. L.; Mitchell, J. M.; Paul, B. D.; Mell, L. D.; Irving, J. J. Anal. Toxicol. 1988, 12, 180-182.
(20) Braithwaite, A.; Cooper, M. Chromatographia 1996, 42, 77-82.
(21) Yoshizako, K.; Hosoya, K.; Kimata, K.; Araki, T.; Tanaka, N. J. Polym. Sci. Pol. Chem. 1997, 35, 2747-2757.
(22) Pesek, J. J.; Tang, V. H. Chromatographia 1994, 39, 649-654.
(23) Mao, Y.; Carr, P. W. LC GC N. Am. 2003, 69-77.
(24) Trammell, B. C.; Ma, L. J.; Luo, H.; Hillmyer, M. A.; Carr, P. W. J. Chromatogr. A 2004, 1060, 61-76.
(25) Nawrocki, J.; Dunlap, C.; Li, J.; Zhao, J.; McNeff, C. V.; McCormick, A.; Carr, P. W. J. Chromatogr. A 2004, 1028, 31-62.
(26) Smith, R. K.; Lewis, P. A.; Weiss, P. S. Prog. Surf. Sci. 2004, 75, 1-68.
(27) Ferretti, S.; Paynter, S.; Russell, D. A.; Sapsford, K. E. Trends Anal. Chem. 2000, 19, 530-540.
(28) Blawas, A. S.; Reichert, W. M. Biomaterials 1998, 19, 595-609.
(29) Netzer, L.; Sagiv, J. J. Am. Chem. Soc. 1983, 105, 674-676.
(30) Sagiv, J. J. Am. Chem. Soc. 1980, 102, 92-98.
(31) Koffas, T. S.; Kim, J.; Lawrence, C. C.; Sormorjai, G. A. Langmuir 2003, 19, 3563-3566.
(32) Kim, J.; Koffas, T. S.; Lawrence, C. C.; Sormorjai, G. A. Langmuir 2004, 20, 4640-4646.
(33) Krishnan, K.; Hill, S. L.; Gelfand, L. S. Proc. Soc. Photo-Opt. Instrum. Eng. 1985, 533, 338-344.
(34) Neivandt, D. J.; Gee, M. L.; Hair, M. L.; Tripp, C. P. J. Phys. Chem. B 1998, 102, 5107-5114.