Preparation and Characterization of Self-assembled Monolayers (SAMs)
Background. Protein-based
biosensors have been popularly used in forensics and biomedical sciences (see
glucose detector in Fig. 1).1,2
 Protein molecules are
Protein molecules are  supposed to detect and identify specific
target compounds (e.g., glucose, drugs of abuse, and their metabolites). More
recently, protein array-based biosensors (proteins are immobilized to specific
locations to form arrays on the surface) have gained a lot of attention due to
their capability to detect multiple target compounds within a single experiment
with enhanced sensitivity and selectivity. Therefore, the preparation of protein arrays with respect to specific
target compounds is the prerequisite to build powerful biosensors.
supposed to detect and identify specific
target compounds (e.g., glucose, drugs of abuse, and their metabolites). More
recently, protein array-based biosensors (proteins are immobilized to specific
locations to form arrays on the surface) have gained a lot of attention due to
their capability to detect multiple target compounds within a single experiment
with enhanced sensitivity and selectivity. Therefore, the preparation of protein arrays with respect to specific
target compounds is the prerequisite to build powerful biosensors.
In order to prepare such protein arrays, several things need to be considered. Since protein arrays are physically defined forms of proteins on the surface, one of the key factors is the ability to immobilize proteins on specified areas with little or no adsorption on the other area. It is also necessary for us to consider which way to attach (or immobilize) proteins to the specified area strongly and uniformly.
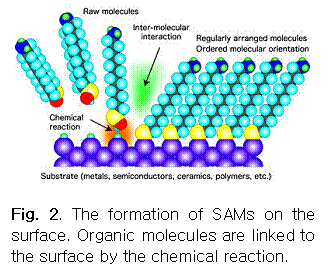
One of the ways to make protein arrays is to conduct protein absorption to patterned surfaces in which one area has a high adsorption affinity and the other has negligible (or no) adsorption affinity. Such functionalities on the solid surface for controlled protein adsorption can be introduced by the use of self-assembled monolayers (SAMs, see Fig. 2).2,3
As the name implies, SAMs are spontaneously formed from the exposure of a solid surface to solutions containing amphiphilic organic molecules with chemical groups that exhibit strong affinities for the substrate (e.g., silanes for silicon oxide and thiols for gold). Since the final surface properties are dictated by the nature and structure of the terminal groups of organic molecules, changing terminal groups gives some control over protein adsorption affinities. This is necessary prior to the preparation of protein arrays.
In
this project, the preparation of two types of SAMs (alkanethiol-based SAMs on gold
and organosilane-based SAMs
on silicon) and their characterization via single reflectance FTIR are
described. More importantly, chemical immobilizations of proteins on the
surface and their characterization will be attempted.
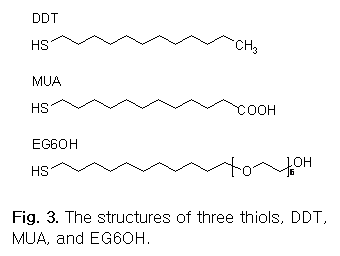 SAMs on Gold substrates. SAMs will be prepared by three thiols,
1-dodecanethiol (DDT), 11-mercaptoundecanoic acid (MUA), and
hydroxyl-terminated (hexaethylene glycol) undecanethiol (EG6OH). These thiols
contain same backbone, but different end groups, methyl (CH3),
carboxyl (COOH), and hydroxyl-terminated hexaethylene
glycol (HO-(CH2CH2O)6)
for DDT, MUA, and EG6OH, respectively (see Fig.
3 for structures).
SAMs on Gold substrates. SAMs will be prepared by three thiols,
1-dodecanethiol (DDT), 11-mercaptoundecanoic acid (MUA), and
hydroxyl-terminated (hexaethylene glycol) undecanethiol (EG6OH). These thiols
contain same backbone, but different end groups, methyl (CH3),
carboxyl (COOH), and hydroxyl-terminated hexaethylene
glycol (HO-(CH2CH2O)6)
for DDT, MUA, and EG6OH, respectively (see Fig.
3 for structures).
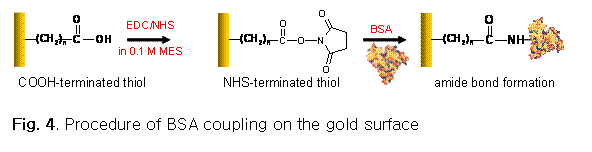
Once MUA is formed, COOH group in MUA will
be converted into N-hydroxylsuccinimide (NHS) group
via chemical reaction using 1-ethyl-3-
Instead of amide coupling between NHS moieties on the surface and primary amine groups in proteins, a hydrazone coupling is also considered. A hydrazone coupling occurs between a hydrazide group (-CO-NH-NH2) and an aldehyde group (-CHO) to form a hydrazone group (-CO-NH-N=CH-).4,6-8 The hydrazide group can be formed from the reaction of NHS group and hydrazine (H2NNH2). The aldehyde group will be prepared by the mild oxidation of primary hydroxyl groups in carbohydrate moieties. Therefore, the coupling is limited to a certain type of protein such as immunoglobulin G (IgG) due to the availability of carbohydrates.
 SAMs on Silicon substrates. Organic
self-assembled monolayers (SAMs)
are of great significance in surface technology since the presence of
chemically bound molecules
render properties of the modified surface entirely different compared to
those of bare substrate.2,9 In particular, the
preparation of SAMs on silicon provides a simple
opportunity to introduce chemically well-defined thin films at the molecular
scale as a prerequisite step in device manufacturing.10-12
SAMs on Silicon substrates. Organic
self-assembled monolayers (SAMs)
are of great significance in surface technology since the presence of
chemically bound molecules
render properties of the modified surface entirely different compared to
those of bare substrate.2,9 In particular, the
preparation of SAMs on silicon provides a simple
opportunity to introduce chemically well-defined thin films at the molecular
scale as a prerequisite step in device manufacturing.10-12
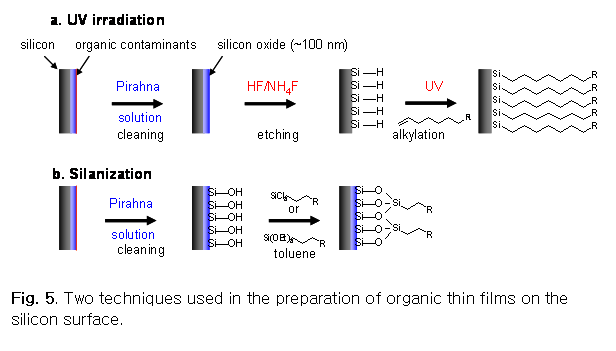
SAMs on silicon substrates can be prepared several ways.
Alkenes will react with clean silicon surface (Si-H)
to form organic monolayers upon UV irradiation (see Fig. 5a).11,12 Alternatively, silanes form 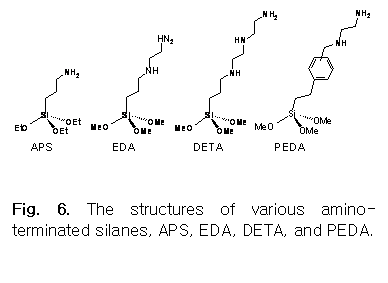 organic thin films on the silicon surface in
solution via silanization reaction (see Fig. 5b).3,13-18 In both cases, organic
molecules (alkenes or silanes) form uniform organic monolayers on the silicon surface via self-assembling.
Variations in terminal groups and structures of organic molecules (alkenes and silanes) have greatly extended the utility of silicon
as a solid substrate by presenting specific chemical and physical properties.
organic thin films on the silicon surface in
solution via silanization reaction (see Fig. 5b).3,13-18 In both cases, organic
molecules (alkenes or silanes) form uniform organic monolayers on the silicon surface via self-assembling.
Variations in terminal groups and structures of organic molecules (alkenes and silanes) have greatly extended the utility of silicon
as a solid substrate by presenting specific chemical and physical properties.
Once formed, terminal groups in SAMs can be modified to introduce chemically reactive moieties on the surface. In this respect, carboxyl- and amine-terminated SAMs, for example, are of interest because further chemical reactions of these groups can facilitate the controlled immobilization of biomolecules via peptide linkage (e.g., proteins)5,19 or phosphoramidite linkage (e.g., oligomer nucleic acids) with an ultimate application to biosensor development.20-22
In this project, four amino-terminated silanes (3-aminopropyltriethoxysilane (APS), N-(2-aminoethyl)-3-aminopropyltrimethoxysilane (EDA), trimethoxysilylpropyldiethylenetriamine (DETA), and m,p-(aminoethyl-aminomethyl)phenethyltrimethoxysilane (PEDA)) will be used for the proposed work (see Fig. 6 for structures). SAMs will be prepared using corresponding silane solutions in anhydrous toluene (ca. 1.0% w/w). SAMs on silicon substrates will be characterized by Fourier transform infrared spectroscopy (FTIR)21 Fourier transform infrared spectroscopy with attenuated total reflection (FTIR-ATR) is especially promising and has proven to be a feasible technique to obtain spectroscopic features regarding organic thin films.23,24
References
(1) MacBeath, G.; Schreiber, S. L. Science 2000, 289, 1760-1763.
(2) Smith, R. K.; Lewis, P. A.; Weiss, P. S. Prog. Surf. Sci. 2004, 75, 1-68.
(3) Sagiv, J. J. Am. Chem. Soc. 1980, 102, 92-98.
(4) Hirota, J.; Michikawa, T.; Natsume, T.; Furuichi, T.; Mikoshiba, K. FEBS Lett. 1999, 456, 322-326.
(5) Jiang, K. Y.; Schadler, L. S.; Siegel, R. W.; Zhang, X. J.; Zhang, H. F.; Terrones, M. J. Mater. Chem. 2004, 14, 37-39.
(6) Xu, Q. G.; Yuan, X. B.; Chang, J. J. Appl. Polym. Sci. 2005, 95, 487-493.
(7) O'shannessy, D. J.; Wilchek, M. Anal. Biochem. 1990, 191, 1-8.
(8) Soltys, P. J.; Etzel, M. R. Biomaterials 2000, 21, 37-48.
(9) Ferretti, S.; Paynter, S.; Russell, D. A.; Sapsford, K. E. Trends Anal. Chem. 2000, 19, 530-540.
(10) Boukherroub, R.; Wayner, D. D. M. J. Am. Chem. Soc. 1999, 121, 11513-11515. .
(11) Linford, M. R.; Fenter, P.; Eisenberger, P. M.; Chidsey, C. E. D. J. Am. Chem. Soc. 1995, 117, 3145-3155.
(12) Strother, T.; Cai, W.; Zhao, X.; Hamers, R. J.; Smith, L. M. J. Am. Chem. Soc. 2000, 122, 1205-1209.
(13) Kurth, D. G.; Bein, T. Langmuir 1993, 9, 2965-2973.
(14) Guo, W.; Ruckenstein, E. Journal of Membrane Science 2003, 215, 141-155.
(15) Meagher, R. J.; Seong, J.; Laibinis, P. E.; Barron, A. E. Electrophoresis 2004, 25, 405-414.
(16) Lee, S. W.; Laibinis, P. E. Biomaterials 1998, 19, 1669-1675.
(17) Banga, R.; Yarwood, J. Langmuir 1995, 11, 618-622.
(18) Barness, Y.; Gershevitz, O.; Sekar, M.; Sukenik, C. N. Langmuir 2000, 16, 247-251.
(19) Patel, N.; Davies, M. C.; Hartshorne, M.; Heaton, R. J.; Roberts, C. J.; Tendler, S. J. B.; Williams, P. M. Langmuir 1997, 13, 6485 -6490.
(20) Chrisey, L. A.; Lee, G. U.; O΅―Ferrall, E. Nucleic Acids Res. 1996, 24, 3031 - 3039.
(21) Saprigin, A. V.; Thomas, C. W.; Dulcey, C. S.; Patterson Jr, C. H.; Spector, M. S. Surf. Interface Anal. 2004, 36, 24-32.
(22) Hooper, A. E.; Werho, D.; Hopson, T.; Palmer, O. Surf. Interface Anal. 2001, 31, 809-814.
(23) Olsen, J. E.; Shimura, F. J. Appl. Phys. 1989, 66, 1353-1358.
(24) Milosevic, M.; Berets, S. L.; Fadeev, A. Y. Appl. Spectrosc. 2003, 57, 724-727.