CHE112
EXAM 4 ___________
Balance the following equations:
1. Fe + HNO3®
Fe(NO3)3 + NO2 +
Fe + 6HNO3®Fe(NO3)3
+ 3NO2 + 3H2O
2. MnO2 + HBr ®
MnO2 + 4HBr ®
MnBr2 + Br2 +2H2O
3. HNO3 + Cu(OH)2® 2HNO3 + Cu(OH)2® Cu(NO3)2 + 2 H2O
4. Sn + HCl® Sn + 2HCl® SnCl2 + H2
5. Zn + H2SO4 (diluted) ®
Zn + H2SO4®
ZnSO4 + H2
6. KClO3®
(thermal decomposition) 2 KClO3®2KCl
+ 3 O2
Give structural formulas for each of the following
compounds:
7. formic acid
8. dichloromethane
9. cyclopentanol
10. dihydrogen phosphate ion
Name the following compounds:
11. CH3CH2CH2OH
propanol
12. H2C=CH-CH=CH2
butadiene
13. CH3Br
bromomethane (methyl bromide)
14-16.
Draw full formulas (show all atoms) in following:
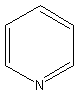
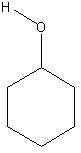

17.Which
kind of organic molecules contains three fragmentsof
organic
acids and glycerol?
Lipids
(fats, triglicerides)
18.
Draw structural formula of alkyne containing two carbon atoms.H-CºC-H
19-20.
How it is possible to make polyethylene from ethanol (ethyl
alcohol)?Showreactions.
CH3-CH2-OH
®
H2C=CH2 + H2O (elimination)
n H2C=CH2®
(-CH2-CH2-) polymerization