Dichromate ion reduces to two chromium(III) ions. This reaction requires 6 electrons and 14 (!) hydrogen ions:
K2Cr2O7 + 6Fe(NH4)2(SO4)2 + 7H2SO4 ®
II. Experimental Procedure.
A. Preparation of a Solution of K2Cr2O7.
Weigh out 1.0-1.2 grams of K2Cr2O7 , transfer into a 250 mL volumetric flask, dissolve this sample in distilled water, and carefully dilute to the mark with additional distilled water. Mix the solution thoroughly by stoppering the flask and inverting several times.
As an alternative, a larger quantity of this solution may be prepared in the stockroom and delivered to the students.
B. Titration of unknown Fe(II) solution
You receive a solution of unknown concentration in 100 mL volumetric flask. Dilute it carefully to the mark.
1. Using a 10 mL pipet, transfer exactly 10.00 mL of an unknown solution into an Erlenmeyer flask.
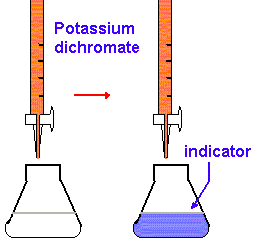
2. Using a graduated cylinder, add 25 mL of 1 M H2SO4 to each flask. Then add 10 mL of the l phosphoric acid solution and 8 drops of sodium diphenylamine sulfonate indicator to the flask. Swirl each flask gently to mix the contents.
3. Fill your buret with the K2Cr2O7 solution and drain out enough so that the liquid level is just below the upper calibration mark and the buret tip is full. Read the initial volume from the calibration scale on the buret. This reading and all other buret readings should be estimated to the nearest 0.01 mL.
4. Titrate the iron solution in the flask. The intense purple color produced by the first drop of excess K2Cr2O7 signals the end point for the titration. Obtain the final volume reading from the calibration scale on the buret.
III. Calculations.
In all calculations we presume that 6 moles Fe(NH4)2(SO4)2 are equivalent to 1 mole K2Cr2O7.
1. Knowing the molarity of your K2Cr2O7 and the volume used in each titration, you can calculate the molarity of your Fe(II) solution as
molarity(Fe(II)) = 6 x molarity(Cr2O7)´volume(Cr2O7)/volume(Fe(II))
2. Knowing the molarity of your Fe(II) solution then allows you to calculate the number of moles of iron (as Fe2+) in your unknown sample and the mass of Fe in g:
Take into account that your total volume is 100 mL
5. Repeat the foregoing calculations for each sample titrated and determine an average value and standard deviation.