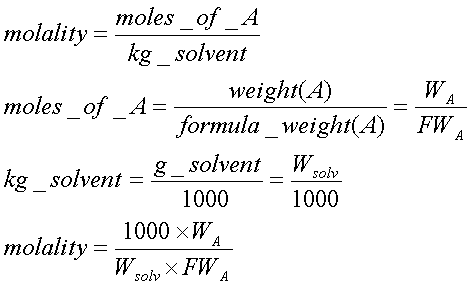
where WA is the mass of compound A, Wsolv is the
mass of solvent, and FWA, is the formula weight of A. Hence,
by substitution of (2) into (1) and subsequent rearrangement we obtain
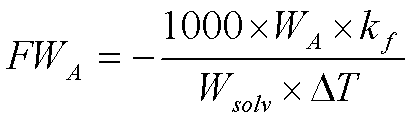 (3)
(3)
Equation (3) tells us that we may calculate the molecular weight of
A (FWA) provided a solution is prepared containing known masses
of solute and solvent (WA and Wsolv), the freezing-point
depression of the solution can be measured, and the value of kf for
the solvent is known. This is a fairly common method for determining the
molecular weights of non-volatile substances and it forms the basis for
this experiment.
You will dissolve an accurately weighed quantity of an unknown solute
in an accurately weight quantity of solvent and measure the freezing-point
depression for the resulting solution. Then, using the freezing-point depression
constant for solvent (Table 1) and equation (3), you will calculate the
molecular weight of the unknown solute.
Sample calculation.
A solution prepared by dissolving 1.53 g of an unknown compound in
5.60 g of naphthalene has a freezing point which is 10.1 oC
lower than the freezing point of pure naphthalene. The freezing-point depression
constant for naphthalene is 6.90 oC/m. What is the molecular
weight of the solute?
- (1000 g/kg)(6.90 oC/m)(1.53 g) / (5.60 g)(- 10.1 oC) = 187 g/mol
II. Experimental Procedure.
1. Pipet exactly (± 0.01 mL) 15
mL of cyclohexane into the 10 cm tube. For cyclohexane, |
|
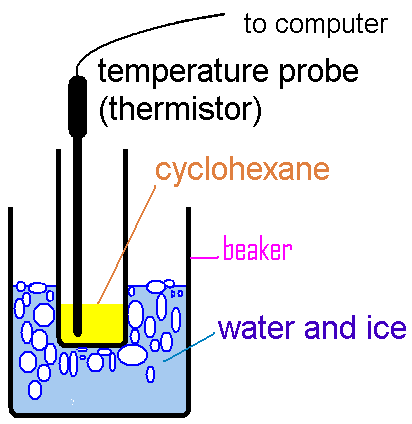
2. Prepare a beaker containing ice and water. Stir it until the temperature is close to freezing. Insert the test tube in a beaker containing ice and water.
3. Place a temperature probe in the test tube. Your setup should be similar to that illustrated in the diagram. |
|
4. Start data collection (Click on "Collect" icon) and
observe the falling temperature of the molten solvent. Gently
stir the liquid with the probe during this period of 5. In the window data, click at "Store latest run".
|
|
7. Melt the Cyclohexane and carefully remove any that is adhering to the walls of the tube.
Accurately weigh out 0.1 g (± 0.001 g) of the unknown compound on a piece of clean weighing paper. Transfer the sample to the tube containing the cyclohexane.
You can use an ultrasonic cleaning bath to help the sample dissolve. Here is a of how this works.
|
(Video)
(Video)
|
8. Repeat steps 3 - 6. Be sure that all of the unknown dissolves
in the cyclohexane.
|
(Video) |
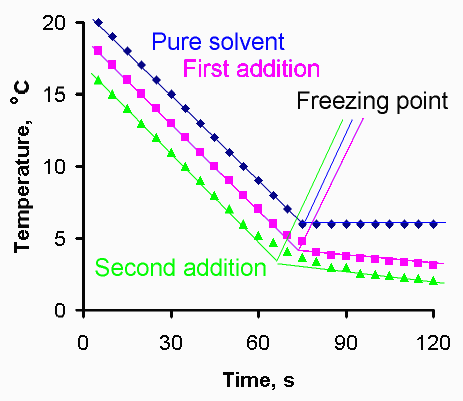
9. Calculate two D Tf values.
10. Calculate the molecular weight of the solute (3 significant figures).
Estimate standard deviation of your result.