|
Introductory remarks
Most of compounds of the above mentioned ions are colored. These colors could
be enhanced in order to give a characteristic effect which would undoubtedly
show the presence of the ion in an unknown mixture.

Cr. The most
stable oxidation states of chromium are Cr(III) and
Cr(VI). Green (or blue, or purple) Cr(III) salts give a precipitate of Cr(OH)3
when they react with bases:
Cr3+ + 3 OH- ®
Cr(OH)3 or CrCl3 + 3 KOH ® Cr(OH)3 + 3 KCl
Chromium hydroxide Cr(OH)3 dissolves in strongly alkaline
solutions forming tetrahydroxochromate(III) ion:
Cr(OH)3 + OH- ®
[Cr(OH)4]-
tetrahydroxochromate(III) can in turn be readily
oxidized to chromate(VI) using hydrogen peroxide:
2 OH- +2[Cr(OH)4]- + 3H2O2® 2CrO42- + 8 H2O

All other 3d transition metal ions for precipitates of appropriate hydroxides
at these conditions. Only Zn(II) forms colorless tetrahydroxozincate [Zn(OH)4]2-
that does not interfere with chromium determination. The overall reaction can
be written as
2Cr3+ + 10 OH- + 3 H2O2
®2CrO42-
+ 8 H2O
or
2 CrCl3 + 10 NaOH + 3 H2O2
®2 Na2CrO4
+ 8 H2O + 6 NaCl
Cr3+: Add an excess of NaOH solution and
some H2O2. Heat the resulting mixture. Filter the
solution and observe the color of the filtrate. If it is yellow, you have CrO42-
ion in filtrate. 
Mn. Almost colorless
(actually slightly pinkish) Mn(II) salts can be oxidized in strongly acidic solution to
give purple permanganate ion MnO4-. Several different
oxidants can be employed for that purpose, among them potassium periodate KIO4:
2Mn2+ + 5IO4- + 3 H2O®2MnO4-
+ 5IO3- + 6 H+
Mn2+: Put 1 droplet of unknown (NO more than 1!!!) solution into a
test tube and dilute it with some H2SO4. Add KIO4
(white solid) and warm gently. The appearance of characteristic purple color
shows the presence of Mn. Very sensitive reaction. 
Fe. The most stable oxidation states of iron are Fe(III) and Fe(II). Fe(III) ions
form yellow or brown ( rusty!) or red compounds with various agents. The
reaction with thiocyanate ion is among the best for
Fe(III) determination:
Fe3+ + 3 SCN- ® Fe(SCN)3
Fe3+: To a drop of unknown solution, add several drops of NH4SCN.
Bloody red color appears if Fe(III) is present. 
Almost
colorless Fe(II) salts form deep blue precipitate with Fe(CN)63-:
3 Fe2+ + 2 Fe(CN)63-®Fe3[Fe(CN)6]2
Fe2+: To a drop of unknown , add several drops of K3Fe(CN)6
. The deep blue color is the indication of Fe2+. 
Co. Most of common cobalt(II) salts
are pink or orange. Some bright blue cobalt (II) compounds form in organic
solvents:
Co2+ + 4 SCN- ® Co(NCS)42-
Co2+: To a drop of unknown solution, add several drops of NH4SCN
in acetone or butanol. The bright blue color is the
indication of Co2+. 
Attention:
the Fe3+ ions need to be masked by NaF
while Cu2+ ions require some sodium thiosulfate to
mask them.
Ni. A very selective
reaction for Ni(II) determination is known for
almost 100 years. It includes the formation of the complex compound:
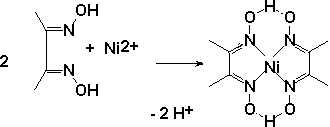
Ni2+: To several drops of unknown, add some tartric
acid , dimethylglyoxime
solution, and then slowly add aqueous ammonia. Drops of ammonia solution must
go by the wall of the test tube. A pink color of nickel glyoximate
appears.
The reaction can be performed on the filtration paper. Add some sodium
phosphate, then unknown, followed by more sodium phosphate and dimethylglyoxime. Expose the resulting spot on the paper
to ammonia vapor. 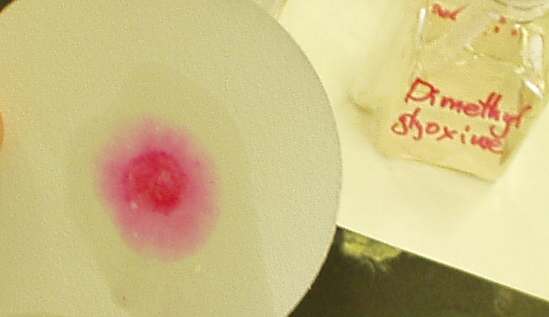
Cu. Blue color of
copper salts is readily visible in concentrated solutions. To observe it in
diluted solutions, you should convert copper into tetraammine
complex:
Cu2+ + 4 NH3 ® [Cu(NH3)4]2+
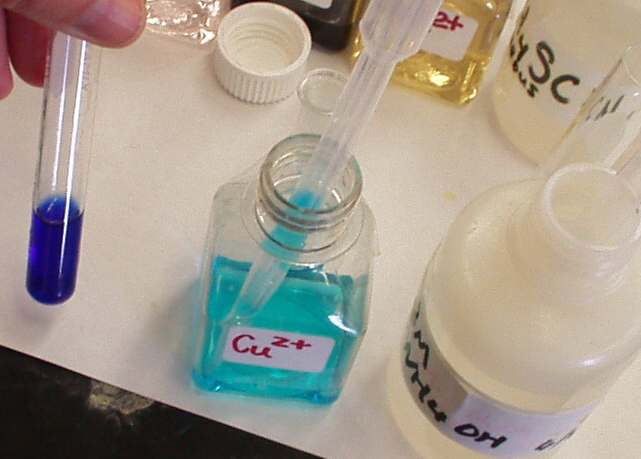 Large quantities of Ni(II) can
interfere with Cu(II) determination because Ni(II) forms blue-violet hexaammino complex [Ni(NH3)6]2+
. Large quantities of Ni(II) can
interfere with Cu(II) determination because Ni(II) forms blue-violet hexaammino complex [Ni(NH3)6]2+
.
Contrary to Ni(II), Cu(II) reacts with iodide forming molecular iodine I2
and copper(I) iodide:
Cu2+ + 4 I- ® 2
CuI + I2
 Cu2+:
To several drops of unknown, add some aqueous ammonia and warm the solution.
Filter the precipitate if any. If the resulting color is blue you have Cu. If
you have lots of Ni, it also can give you similar effect. In this case,
neutralize the filtrate with acetic acid and add some KI. The appearance of
brown I2 indicates that you have Cu as well. Cu2+:
To several drops of unknown, add some aqueous ammonia and warm the solution.
Filter the precipitate if any. If the resulting color is blue you have Cu. If
you have lots of Ni, it also can give you similar effect. In this case,
neutralize the filtrate with acetic acid and add some KI. The appearance of
brown I2 indicates that you have Cu as well.
|
