3. Quantitative measurements of inorganic anions in solutions.
There were numerous successful attempts to apply Raman spectroscopy for quantitation of various polyatomic species [22]. The relatively low sensitivity of quantitative methods based on Raman scattering prohibits their application in trace analysis. At the same time, high selectivity allows the use of Raman spectra for determination of relatively large quantities of various analytes even in complex mixtures. Here we present several possible applications of Raman spectra for the quantitative assay for several oxoanions.
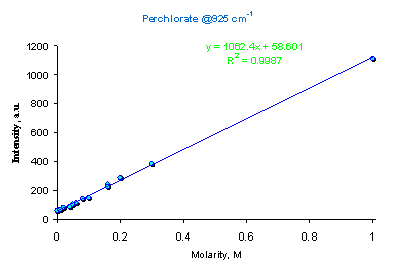
For relatively low amounts of analyte (0.1-10%), a good linearity of calibration plot can be expected. Indeed, in all cases tested here the calibration was a straight line with R>0.99 (Figure 5).
Figure 5. Calibration plot for perchlorate ion in water using intensity of Raman peak at 925 cm-1.
3.1. Sulfate ion in water.
High concentration of sulfate is usually unwanted
in drinking water. For example, more than a third of the farms in the southern
and central parts of Saskatchewan (
Direct detection of sulfate is possible at concentrations above 0.5 mg/mL. This makes Raman spectroscopy suitable for direct determination of sulfate in seawater, salt lakes, and high-sulfate ground water. Most of water samples require pre-concentration which can be easily achieved by simple evaporation. 10-100 mL of water can be quickly evaporated to a small volume (around 1 mL) which is suitable for Raman measurements. Perchlorate ion was recommended as an internal standard for high-resolution Raman determination of sulfate [18].
Experimental Procedure: Measure Raman spectrum of the solution and subtract the spectrum of pure water. If sulfate peak (980 cm-1) is detectable, its concentration is around 1 mg/mL or higher, and it can be directly calculated from the calibration plot. For highly mineralized water samples ([SO42-]> 0.1 mg/mL), take a 20 mL sample, carefully acidify to pH 5 if necessary, and evaporate to the volume of 2 mL. Measure the Raman spectrum and calculate sulfate concentration. For water samples with lower mineralization (for example, usual tap water), evaporation of larger samples, 250-500 mL, is required. A known amount of internal standard, 0.10 mL of 1 M NaClO4, should be added before evaporation if an internal standard method is applied.
Several examples of sulfate determinations
in natural water are shown in Figure 6. The sulfate peak in the spectrum of
a seawater sample is clearly visible, and no preconcentration is necessary.
Two samples of sulfate-hydrocarbonate mineral water were analyzed after 10-fold
preconcentration. Tap water (Buffalo, New York) and mineral water with low
sulfate content (Evian,
This Raman procedure can serve as a quick semi-quantitative test for sulfate in drinking and technical water: if a peak at 980 cm-1 is visible, the sample does not comply with the WHO drinking water standard of 0.5 mg/mL.
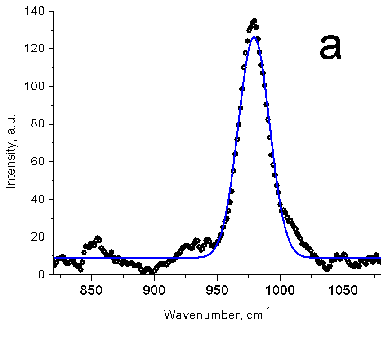
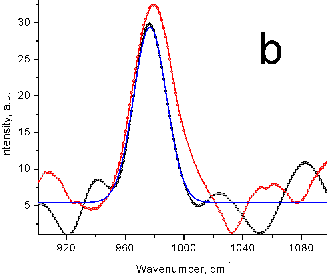
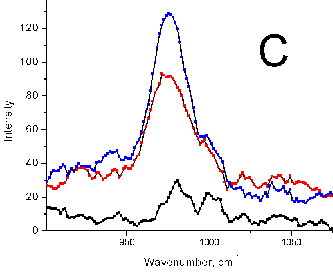
Figure 6 (a) Raman peak of sulfate ion in a standard
solution (10 mg/mL SO42-). (b) Sea water samples (red
and black) with a Gaussian fit. No preconcentration. (c) Sulfate ion in spectra
of natural ground water (Mirgorod source,
3.2. Nitrate and nitrite.
Nitrate and nitrite ions can be easily detected at 1 mg/mL level. There is no spectral interference between these two ions because of significant distance between the most prominent Raman peaks. Chloride ion presence also does not affect the detection. This differs favorably from the usual spectrophotometric procedures which suffer from significant mutual interference and can fail in the presence of high amounts of chloride ion. The first description of Raman determination of nitrate can be found in [19]. High resolution UV resonance Raman procedure was described [20]; sub-ppm quantities of nitrate and perchlorate were successfully measured by Raman spectrometer after electrophoretic preconcentration [21].
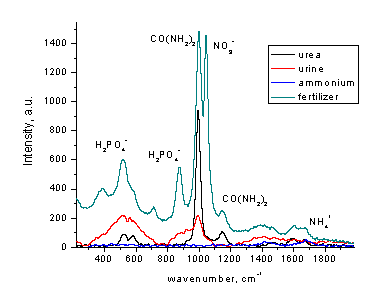
Solutions of various fertilizers present an example suitable for direct determination of nitrate without pretreatment (Figure 7). Other components of fertilizer mixture shown, urea and phosphate ion, can also be evaluated from separate calibrations. It is worth mentioning that Raman procedures for urea [22] and phosphate [22] determination were published. Carbamate ion may interfere with low-resolution Raman detection of nitrate.
Figure 7. Raman spectra of various nitrogen-containing fertilizers.
3.3. Perchlorate Determination.
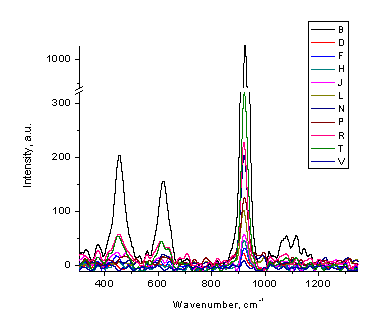
Raman spectroscopy was suggested as a suitable method for perchlorate quantitation [23,24]. Measurements of the peak at 925 cm-1 resulted in a good quality calibration plot (Figure 5). Raman signal is easily visible at 1 mg/mL concentration (Figure 8). Despite the apparent success, we have not yet found a suitable educational experiment for perchlorate determination.
Figure 8. Low resolution Raman spectra of sodium perchlorate solutions of different concentrations (1,2,4,5,6,8,10,16,20,30,100 mg/mL)