4. Identification and determination of organic molecules.
Organic chemistry represents the most common application of Raman spectra. Here we describe several examples suitable for the undergraduate curriculum.
4.1. Determination of components in an industrial xylene sample
A commercial mixture of xylenes contains all three isomers of xylene, ethylbenzene, and less than 1% of toluene and other aromatic hydrocarbons. This is one of the most common examples of Raman spectroscopy use, it was described in the first publications on quantitative Raman analysis [19]. For the undergraduate lab, we compare three methods suitable for determination of components in the four-component mixture: FTIR, GC, and Raman.
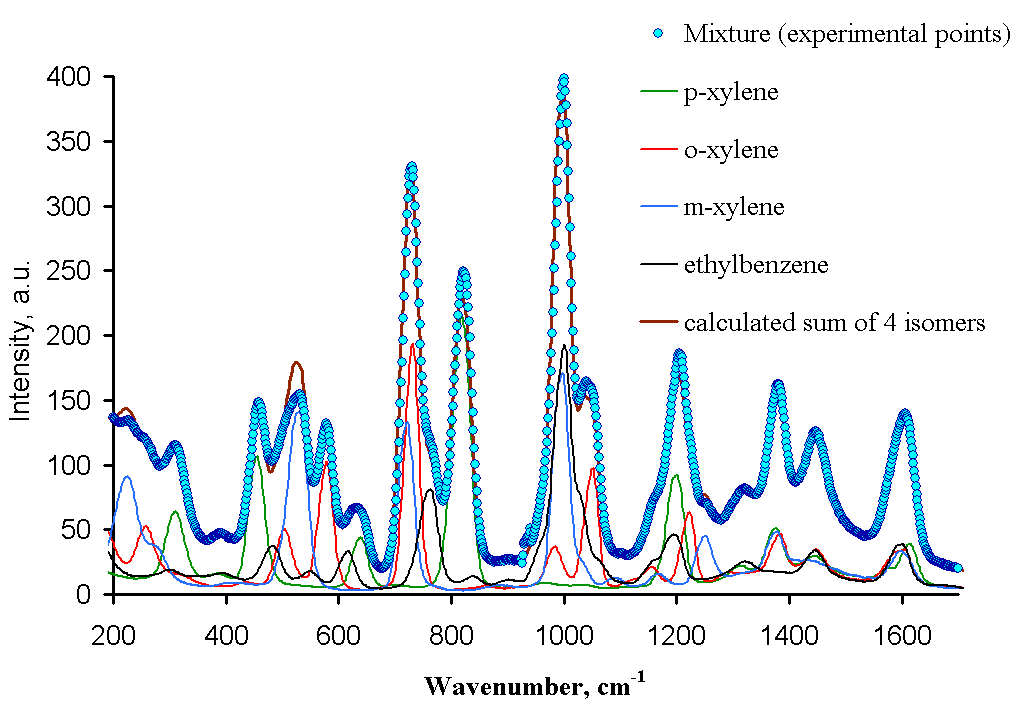
Procedure: Put a cuvette containing an analyzed solution in a sample compartment, cover it carefully with the cap, and record the Raman spectrum (recommended scan time is 12 s). Repeat the same measurements for o-, m-, and p-xylene, and for an unknown mixture of isomers. Save all resulting spectra (an example of such spectra is shown in Figure 9).
Using ACD UV-IR Processor program, open each resulting file and process them in 200-1000 cm-1 range. Find peaks that are characteristic for each isomer and do not overlap with peaks from other isomers. From intensities of the peaks in the mixture and in pure isomers, estimate the composition of the xylene mixture. Compare IR and Raman spectra of xylene isomers (IR spectra are available from the corresponding FTIR experiment). Students can also perform the GC separation of xylene mixture with subsequent quantitation. The comparison of the results from three independent analytical methods provides an important experience in validation of the assay.
Figure 9. Low resolution Raman spectra of ethylbenzene and three isomers of xylene.
Usually students’ results are in ±2-3% agreement with the actual composition of the mixture.
4.2. Identification of alcohols
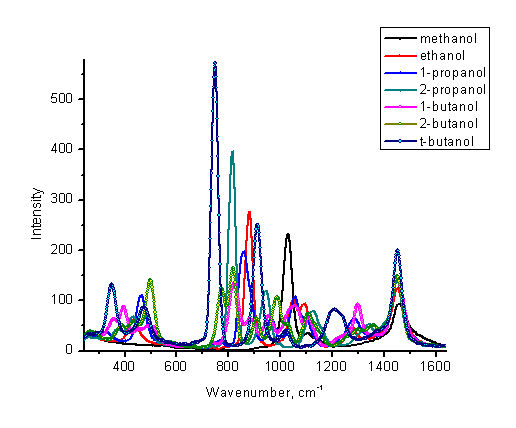
Common organic solvents (alcohols, acetone, acetonitrile, etc.) represent another experiment set of the samples for the identification. If the Raman database is not available, the instructor may want to reduce the list of possible unknown compounds to alcohols with one-six carbon atoms (see Figure 10). In such a limited case visual comparison with the library spectra can be easily accomplished.
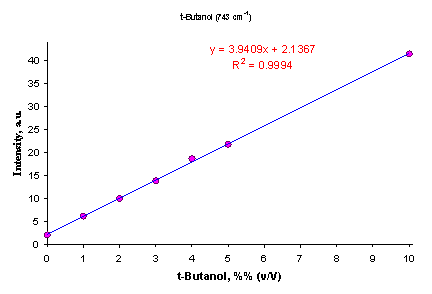
Quantitation of water-soluble alcohols in aqueous solution can be easily achieved in a wide range of concentrations (Figure 11).
Figure 10. Low resolution Raman spectra of some alcohols.
4.3. Identification of sugars in aqueous solutions.
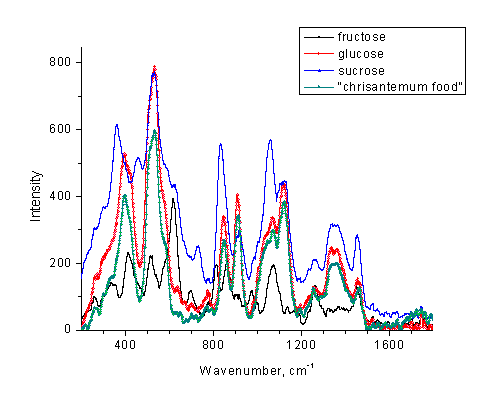
A similar setup can be recommended for sugar identification. In this case, the aqueous solutions are employed making the analytical problem slightly more difficult. An example of an unknown sugar identification in a commercial product is shown in Figure 12.
Figure 11. Calibration curve for t-butanol (2-methyl-2-propanol) determination in water using 743 cm-1 Raman peak.
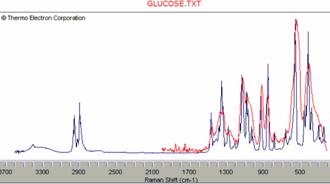
If a commercial Raman database is available, the identification problem becomes more straightforward and less challenging. To our experience, high resolution databases can be directly used with low resolution spectra. Two examples of such searches are presented in Figure 13. A spectrum of glucose in an aqueous solution, after subtraction of water scattering, instantly resulted in a correct output. The same was true for pure t-amyl alcohol.
Figure 12. Low
resolution Raman spectra of several sugar solutions in water and a sample
of a glucose-based aqueous solution (“chrisantemum
food” from 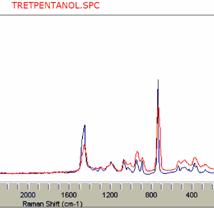 WalMart).
WalMart).
Figure 13. Low resolution Raman spectra of glucose and t-pentanol (red) compared with database high resolution spectra of the same compounds (blue). The low resolution did not prevent the search engine from performing a successful search (www.ftirsearch.com).
Conclusions.
From the procedures presented in this work, it is apparent that low resolution Raman data can be quite useful in an undergraduate laboratory. With a low resolution instrument, it is possible to identify chemical compounds in solids and in solutions, organic as well as inorganic molecules. One can build a calibration curve and quantify the amount of analyte in a wide range of concentrations (often 3 orders of magnitude). All this makes a low resolution Raman instrument a necessary and an affordable tool like FTIR, UV, and NMR spectrometers.
Acknowledgements
This laboratory setup was tested with chemistry and forensic chemistry students at SUNY College at Buffalo in 2004 and 2005; without their patience and helpful comments this project could not be accomplished. The financial support from OceanOptics Inc (educational grant B132) and SUNY SCAP is gratefully acknowledged.