Acetic acid reacts with sodium hydroxide to make sodium acetate and water:
HOAc + NaOH ® NaOAc + H2O
or, HOAc + OH- ® OAc- + H2O
Materials.
Standard NaOH solution.
Indicators: phenolphtalein, methyl orange.
pH-meter with pH-sensitive electrodes.
Buret
Procedure.
You will be provided with the sample of acetic acid (HOAc) in a 100
mL volumetric flask. Carefully dilute the sample to the mark with deionized
water. Mix the resulting solution.
1)Pipet a 10 mL of solution into a Erlenmeyer flask. Add 3 drops of
phenolphtalein and titrate with standard 0.100 M NaOH to the pink end point.
Near the end point, the indicator color temporarily appears as titrant
is added. When you recognize this, you should slow down the rate of addition
and estimate the end point to within 1-2 drops.
2)Repeat the same titration. This time you already know the result,
so you can do it more carefully.
3) Now comes the pH-metric titration. You should still add some phenolphtalein
to the solution. Write pH of the solution before the addition of any NaOH.
Add 2 mL of NaOH, and write the resulting pH value, the next 2 mL and so
on, until you are coming as close as 2 mL to your expected end point (this
is the volume you were receiving while titrating with phenolphtalein).
Staarting from here, you add 0.1 mL before each reading. Detect the point
when the color appears. Continue the addition at the same rate for 1 mL
more, than take 2-3 readings with 2 mL steps.
4) Repeat the same pH-metric titration using methyl orange as indicator.
Is the pH range of the color change the same as with phenolphtalein?
Calculations.
1. Plot the results of your potentiometric titration. The point of
the highest jump of pH is your pH-metric end point. Compare it with your
end point from phenolphtalein color.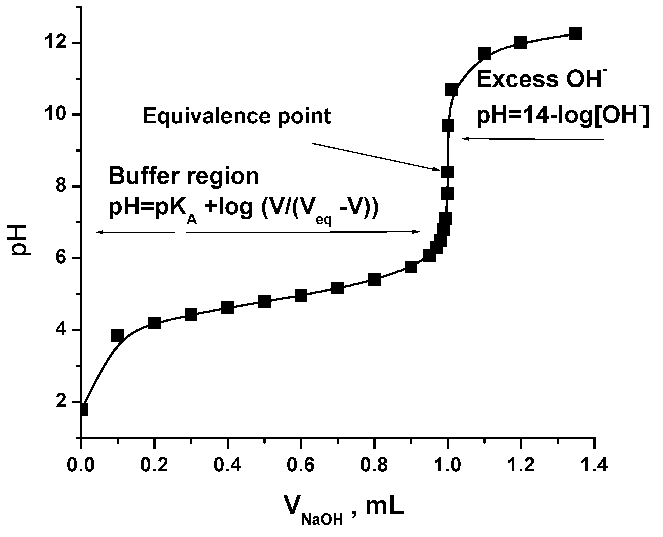
2.Use your data to calculate concentration of the acetic acid in you
100 mL flask. At the equivalence point, number of moles of HOAc before
titrations is exactly equal to the number of moles of NaOH you have added.
NHOAc = NNaOH
Number of moles of any compound in solution NX can be written
as
NX = CX VX, where CX is
molarity, and VX is volume. Thus,
CHOAc VHOAc = CNaOH VNaOH,
and CHOAc = CNaOH VNaOH/ VHOAc
3. Calculate the total amount of acetic acid in your 100 mL in grams:
mHOAc = FWHOAc CHOAc Vflask
(FWHOAc = 60, Vflask = 100 mL = 0.1 L)
4. Calculate the pK of acetic acid. For doing that, use 6-8
points of your pH-metric titration before the end point.
From Henderson equation,
pH = pK + log ([OAc-]/[HOAc])
or pK = pH + log ([HOAc]/[OAc-]
pK = pH + log ((Vend – V)/V), where V is the
volume of NaOH added at each point, and Vend is the volume
of the ending point.
Average the pK values you receive.
5. From pK value you may estimate the theoretical pH of the end
point.
At the theoretical point of equivalence you have only NaOAc and water.
Number of moles of NaOAc is: N = CNaOH VNaOH
total volume of the solution at the end point is the sum of volumes
of acetic acid (10 mL volume you have pipeted) and sodium hydroxide you
have added from the buret (VNaOH). Thus, the concentration
of sodium acetate at the end point is
CNaOAc = CNaOH VNaOH /(10 mL +
VNaOH)
sodium acetate is a Broensted base because it can react with a proton
H+. For weak bases, [OH-] = Ö
KB C.
You can calculate KB from pK value that you
have received from pH-metric titration:
KB´K = 10-14.
Thus, KB = 10-14/ 10-pK or KB
= 10pK-14
Now you can place KB and C into the equation above to achieve
the concentartion of hydroxide OH- at the end point.
pOH = -log[OH-] and pH = 14-pOH
Multi step calculations from above can be done in one step using concentration
C
for sodium acetate at the end point and pK for acetic acid:
pH= 7+0.5´ pK+0.5´
logC
Compare your estimate for the end point with the results of calculations.
Your results can be written in such format:
Experiment 3.
Name _______________________ Date _____________
Volume of NaOH
Titration No.1 _______ mL
Titration No.2 _______ mL
Titration No.3 _______ mL
Titration No.4 _______ mL
Average _________ mL Standard deviation _________ This is your Vend (see table below)
Concentration of acetic acid _____________
Mass of acetic acid in the flask___________
pH-metric titration