This experiment outlines the techniques necessary to determine the equilibrium constant for the formation of an iron(III) thiocyanate complex ion (FeSCN2+) from Fe3+ and SCN- . The quantitative preparation of several solutions and subsequent measurement of the solution absorbance using a spectrophotometer are the techniques that will be used in this experiment. The absorbance measurement gives the concentration of FeSCN2+. The concentrations of Fe3+ and SCN- are obtained as the difference between the initial concentration and the concentration consumed by the formation of the FeSCN2+. The combined concentrations will be used to calculate an equilibrium constant for the formation of the complex.
The reaction for the formation of the dark red FeSCN2+ complex ion is very simple:
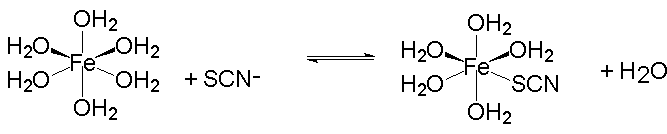
It has an equilibrium constant, K, given by:
Starting with known amounts of iron(III) and thiocyanate, and measuring the amount of FeSCN2+ ion formed at equilibrium, one can calculate the equilibrium amounts of iron(III) and thiocyanate ions. From a knowledge of the equilibrium amounts of all three ions, the equilibrium constant for the reaction may be calculated.
II. Experimental Procedure.
The handling of the instrument will be demonstrated.
Ideally the five equilibrium constants obtained from the five sets of data should be the same. How closely they actually match will be a function of the care you take in carrying the experiment.
It is very important that you understand what you are doing at all times. Proper labeling should help.
A. Standard Solution.
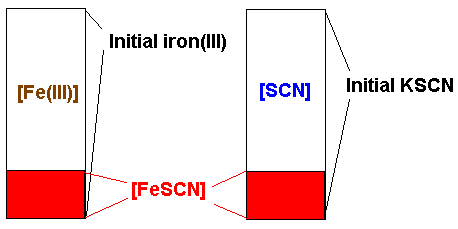
The instrument must be calibrated by a solution in which the concentration of the complex ion is known. This is accomplished by preparing one solution where the starting concentration of iron(III) ions exceeds the starting concentration of thiocyanate ions by two orders of magnitude. Under these conditions, of excess iron, it may be safety assumed that all the original thiocyanate will have been used to form the complex ion (LeChâtelier's principle). Thus the concentration of the complex may be assumed to be equal to the initial concentration of thiocyanate. The concentration of the complex in all the other solutions is then determined by the instrument as a fraction of the concentration of the complex in this, the standard solution.
Pipet 5.0 mL of 0.10 M iron(III) nitrate into each of five 150
mm test tube. Add the following amounts of KSCN and diluted nitric acid
to each of the tubes:
#1 0 mL KSCN and 5 mL nitric acid
#2 0.2 mL KSCN and 4.8 mL nitric acid
#3 0.4 mL KSCN and 4.6 mL nitric acid
#4 0.6 mL KSCN and 4.4 mL nitric acid
#5 0.8 mL KSCN and 4.6 mL nitric acid
Mix them well. Label it. This is your calibration set of solutions.
It is assumed that the concentration of the FeSCN2+ complex
in this solution is exactly equal to total concentration of SCN.
Using the EXCEL program, plot the Absorbance (A) as a
function of thiocyanate concentration; this is your calibration
curve. The trend line should be a straight line with the slope of e
and
intercept b
A=e C+b
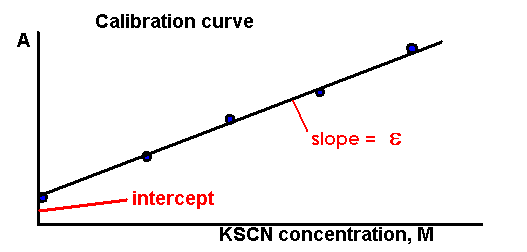
You will use the value of e in
further calculations.
B. Working Solutions.
Label five 150 mm test tubes from 1 to 5. Pipet 5.0 mL of 2.0 mM
Fe3+ into each.
Add the following amounts of KSCN and diluted nitric acid to each of
the tubes:
#1 0.5 mL KSCN and 4.5 mL nitric acid
#2 1 mL KSCN and 4 mL nitric acid
#3 2 mL KSCN and 3 mL nitric acid
#4 3 mL KSCN and 2 mL nitric acid
#5 4 mL KSCN and 1 mL nitric acid
Total volume in each tube is 10 ml (check it!).
Measure absorbance of each solution.
C. Determination of Absorbance
The Spectronic 20 spectrophotometer will be used to measure the amount
of light being absorbed at 450 nm, the wavelength at which the thiocyanatoiron(III)
complex absorbs visible light. Instrument controls will be demonstrated
by your instructor. Connect the instrument to a 115 V AC outlet, and let
it warm-up for 10-15 minutes.
.

The instrument must be calibrated. Set the wavelength to 450 nm with the WAVELENGTH control.
With nothing in the CELL COMPARTMENT, use the DARK CURRENT control (the
same control that turns the instrument on and off) to set the instrument
to read 0% Transmittance (black scale).

Fill a cuvet with deionized water, and dry the outside and wipe it
clean of fingerprints with Kimwipe. Insert the test tube into the CELL
COMPARTMENT as far as it will go. Set the instrument to read 100% Transmittance
with the LIGHT control. The instrument is now calibrated. The settings
of the controls must not be changed from now on, or you will have to recalibrate.
Fill another cuvet with your solution.. Wipe the outside with tissue
and then insert it into the CELL COMPARTMENT (after removing the test tube
containing the deionized water, of course). Determine the absorbance and
record it.
CALCULATIONS
Calculate initial concentrations of iron and of thiocyanate in each
of your five solutions.
Use your calibration to determine the concentration of FeSCN2+
[ FeSCN2+]= A/e
Subtract the [ FeSCN2+] from the initial concentration
of iron: this is your concentration of Fe3+ at equilibrium.
Subtract the [ FeSCN2+] from the initial concentration
of thiocyanate: this is your concentration of SCN- at
equilibrium.
Put the concentrations you have calculated in equation