Reducing agents such as ascorbic acid (vitamin C) are titrated directly with standard iodine solution in the presence of starch, until reaching the intense blue end point:
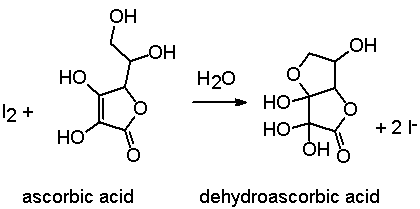
One mole of ascorbic acid (F.W.= 176.13) reacts with one mole of iodine.
Procedure:
You will need a standard solution of iodine. Fill the buret with this
solution and write its concentration from the label in your notebook.
Your unknown solution is placed in 100-mL volumetric flask. Dilute
the unknown to the mark with deionized water. Using a 25 mL pipet, transfer
an aliquot part of the solution into an Erlenmeyer flask. Add some colorless
starch solution, and titrate until the intense blue color.
Repeat the same titration two times more.
Calculations:
To obtain the mass of ascorbic acid in unknown, you need to find molarity
of the ascorbic acid:
CHA = CI2 x VI2/ V HA
mHA = FWHA x C HA x (total volume)
In your case, (total volume)=100 mL= 0.100 L and FW=176.13 g/mol.
Therefore,
mHA = 176.13 x0.100 x CI2 x VI2/V
HA = 17.613 x CI2 x VI2/V HA
Report
Concentration of iodine (copy from the label!): __________
Volume of your pipet _____25.00 mL
Volume of iodine used for titration
# 1 ___________ # 2______________ # 3_____________( mL)
Mass of ascorbic acid
# 1 ___________ # 2______________ # 3______________(g)
Average mass_____________ g
Standard deviation _____________g